Sorting Materials into Groups
All things are made of one or more materials. The same thing can also be made of different materials.
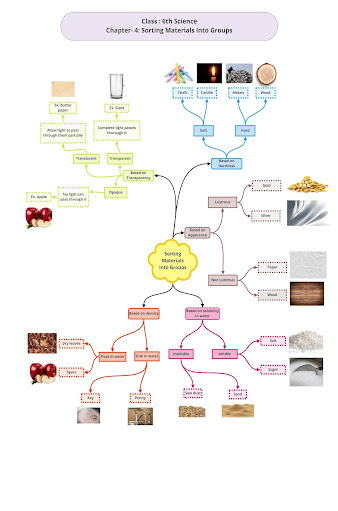
It may be man-made or naturally occurring. Materials can be classified on the basis of many criteria. The sorting of objects into groups with each group having its own characteristics is called the classification of objects.
The substances can also be grouped by the similarity of the materials used to make them
Table 1 shows the object and the materials used to make it.
Table 2 shows the material and the objects made by it.
Table 3 shows a single object made up of different types of materials.
Classification of Materials
Classification of materials is based on the similarities and differences between different materials. It has the following advantages:
It makes it easier and convenient to locate a variety of materials and to work with them.
It also helps in better understanding of materials. This is because if we know the properties of any one member of the group, we can get an idea of the properties of the other members of the same group.
Materials can be classified on the basis of the following properties:
Appearance
The appearance varies from one material to the other. Colour, texture, roughness and other parameters contribute to the appearance of materials. Materials with a shiny surface are called lustrous (gold, silver, copper), whereas those which have a dull appearance are called non-lustrous (paper, cardboard, chalk).
Lustre: Some materials are lustrous(shiny) such as metals. While others are not shiny, The metals can lose their shine because of the presence of rust on them.
Hardness and Softness
Materials which cannot be easily compressed, cut, bent (moulded) or scratched are called hard materials. Examples: Iron, steel, wood, stone
Transparency
• Some substances that allow us to see pass through them are called transparent substances. The light can easily pass through them. The common examples of transparent objects are air, glass and some plastics
• The objects that allow us to see but not clearly through them are called translucent objects. The examples of translucent objects are butter paper, tissue paper and thin plastic etc.
• The objects that do not allow us to see pass through them are called opaque objects. When the light falls on an opaque object, it does pass through it, meanwhile, it forms a shadow. Some examples of opaque objects are wood, human, stone and trees.
• A solution is a mixture of homogeneous (same components) or heterogeneous(different components).
• The material that is present in a small amount in a solution is called the solute. The solute can be dissolved or cannot be dissolved in a solution. For example, sugar can be dissolved in the water, but pieces of rock cannot.
• The solute that can be dissolved in a solution is called soluble substances. While some other substances that are not dissolved in a solution called insoluble substances.
• If the mixture of liquids successfully mix then it is called miscible liquids. While the liquids that do not mix with each other are called immiscible liquids.
• Table 4 shows the solubility test of various materials in the water.
Texture
• The feeling of roughness and smoothness while touching any object is called its texture.
• The smooth objects have plane surfaces and no bumps or ridges. For example, flowers and baby’s skin etc.
• The rough objects have bumps or ridges and irregular surfaces. For example, tree-bark
Conductivity
• The substances that pass the electricity completely are called good conductors. For example, metals.
• The substances that pass the electricity partially are called poor conductors. For example, graphite.
• The substances that do not pass the electricity are called insulators. For example, wood.
Density and floatation
• The mass per unit volume of a substance is called density.
• The objects that have lower density than the water can float on it. For example, ice.
• The objects that have higher density than the water sink to it. For example, metals.
Malleability
It is a property of substances that can be changed into thin sheets while beating up. For example, metals like gold and silver are beaten up into thin sheets with a hammer.
Ductility
It is a property of metals that allow them to change into wires. For example, the filament of an electric bulb is made up of tungsten metal.
• Anything which occupies mass and space is called matter.
• All the substances present in the universe are made up of matter.
Composition of matter
• Many atoms or a few atoms linked together to form matter.
• The smallest unit of a matter is called an atom.
• The same atoms linked together to form a molecule.
States Of Matter
The existence of a matter in different forms is known as states of matter.
There are three states of matter:
a) Solid State
• The particles of the solid-state substances are closely packed.
• The intermolecular force of attraction between the particles in very high.
• The particles are fixed at their places, only vibrate if they get higher energy.
• The solid-state objects have definite shape and size.
• They also have definite volume.
b) Liquid State
• The particles of liquid have spaces between them.
• The intermolecular force of attraction is high but not as the particles of solid.
• The particles of liquid are continuously moving in random directions, and while moving, they collide with each other.
• The liquid substances take definite shape in a container.
• They have definite volume.
c) Gas State
• The particles of gaseous state are loosely packed.
• The intermolecular force of attraction is very low.
• The particles can move freely in any direction.
• They do not have definite shape, size, and volume.
• They can be compressed in a cylinder. For example, Liquid Petroleum Gas s(LPG) and Compressed Natural Gas.
Opacity
Materials through which we can see clearly are called transparent materials. Examples: Glass, air.
Important Questions
Multiple Choice Questions:
Question 1. materials can be used for made up more than one things,
(a) Same
(b) Different
(c) Shiny
(d) None of these
Question 2. How do we choose a material to make an object?
(a) depending on its properties
(b) depending one its colours
(c) depending on its shape
(d) none of these
Question 3. Newspaper, notebook, books and calendars etc. are made by:
(a) iron
(b) wood
(c) paper
(d) none of these
Question 4. Iron, aluminium and gold have appearance.
(a) shining
(b) rough
(c) non-shining
(d) none of these
Question 5. Metals which have a luster are called:
(a) none-lustrous materials
(b) lustrous materials
(c) rough
(d) none of these
Question 6. Wood and stone is materials.
(a) lustrous
(b) non-lustrous
(c) smooth
(d) none of these
Question 7. We see luster on the freshly cut of the wire.
(a) surface
(b) length
(c) both (a) and (b)
(d) none of these
Question 8. A substance dissolve in water is:
(a) sand
(b) chalk
(c) wax
(d) sugar
Question 9. How does aquatic animals survive in water?
(a) due to oxygen gas dissolved in water
(b) due to carbon dioxide gas dissolved in water
(c) they feel very warmth
(d) none of these
Question 10. An object that floats in water is:
(a) wood
(b) sugar
(c) iron nail
(d) none of these
Question 11. An object that sinks in water:
(a) wax
(b) crystals
(c) any oil
(d) none of these
Question 12. A liquid that mixes well in water is:
(a) vinegar
(b) oil (mustard)
(c) glycerin
(d) none of these
Question 13. A liquid that does not mixes well in water is:
(a) lemon juice
(b) vinegar
(c) glycerin
(d) all of these
Question 14. The substance which dissolves in water are called:
(a) soluble
(b) insoluble
(c) miscible
(d) immiscible
Question 15. The substance which does not dissolve in water are called:
(a) soluble
(b) insoluble
(c) miscible
(d) immiscible
Very Short Question:
1. Why do we need to group materials? Give one reason.
2. Suggest two bases on which we can group objects.
3. Is a substance which can be compressed soft or hard?
4. Select a lustrous material out of the following substances:
5. Which material is generally used for making pens? Wood, aluminium, plastic, cotton
6. Is oil soluble in water?
7. Name two objects which are made from opaque materials.
8. What is common between salt and sand?
9. List three liquids which are transparent.
10. Write two substances which are made from leather.
Short Questions:
1. Write any four properties of materials.
2. Why is a tumbler not made with a piece of cloth?
3. What are the similarities between iron, copper and aluminium?
4. Mention some materials which are made up of paper.
5. Why is water important for our body?
6. What is the basis for sorting materials?
7. What is the reason for grouping materials?
8. Metals have luster (shine). Give reason why some metal articles become dull and lose their shine.
Long Questions:
1. ‘Grouping of objects helps the shopkeeper.’ Justify the statement.
2. Describe an experiment to prove that water is transparent.
3. Write an experiment to show that our palm is translucent.
4. How can you show that some solids like sugar, salt are soluble in water whereas solids like chalk powder and sand are not soluble in water?
Answer Key-
Multiple Choice Answers:
1. (a) Same
2. (a) depending on its properties
3. (a) iron
4. (a) shining
5. (b) lustrous materials
6. (b) non-lustrous
7. (a) surface
8. (d) sugar
9. (a) due to oxygen gas dissolved in water
10. (a) wood
11. (b) crystals
12. (a) vinegar
13. (c) glycerin
14. (a) soluble
15. (b) insoluble
Very Short Answers:
1. Answer: We often group materials for our convenience. It helps to describe their properties.
2. Answer:
(i) Material used in making the object, e.g., wood or metal/plastic.
(ii) Material of the object is soft or hard, or substance is soluble or insoluble in water.
3. Answer: Soft.
4. Answer: Aluminium.
5. Answer: Plastic or metal.
6. Answer: Oil does not dissolve in water, so it is insoluble in water but floats on the surface of water.
7. Answer: Wooden doors, blackboard/steel plate.
8. Answer: Both have mass and are in solid state.
9. Ans. Water, alcohol, and Acetone/Benzene.
10. Answer: Belt and shoes.
Short Answer:
1. Answer:
(a) Appearance
(b) Hardness
(c) Solubility
(d) Float or sink in water
(e) Transparency
2. Answer: We use tumblers made of glass, plastic, and metal to keep a liquid. These substances can hold a liquid.
A tumbler made of cloth cannot hold a liquid because:
(i) Cloth piece is not hard enough to hold liquids and
(ii) Cloth piece has very minute pores through which the liquid oozes out.
3. Answer:
(a) They all have luster,
(b) They are all metals,
(c) They are hard.
4. Answer: Books, notebooks, newspapers, toys, calendars, etc.
5. Answer: Water can dissolve a large number of substances, so it is needed by the body. It is also major part of our body cells.
6. Answer: Materials are grouped on the basis of similarities or dissimilarities in their properties.
7. Answer: Materials are grouped for our convenience to study their properties and also observe any patterns in these properties.
8. Answer: Metals when exposed to air react with moisture and gases present in it, thereby forming a dull layer of some other compound on it.
Long Answer:
1. Answer: Proper grouping of objects helps shopkeeper in the following ways:
(i) He can locate the required object easily and quickly.
(ii) He can easily come to know what stocks are going to finish and he should purchase them for his customers.
2. Answer: Take a beaker half-filled with clean water. Put a coin in beaker of water.
Place the beaker undisturbed for a few minutes where enough light is present. Now, observe the coin immersed in water from the top of the beaker. Are you able to see the coin? You can clearly see the coin immersed in water. This proves that water is a transparent liquid.
3. Answer: Cover the glass of a torch with your palm at a dark place. Switch on the torch and observe from the other side of palm. We see that the light of torch passes through palm but not clearly. This experiment shows that our palm becomes translucent when a strong beam of light passes through it.
4. Answer: Collect samples of sugar, salt, chalk powder and sand. Take four beakers. Fill each one of them about two-third with water. Add a teaspoonful of sugar to the first beaker, salt to the second, chalk powder to the third and sand to the fourth. Stir the contents of each beaker with a spoon/stirrer.
Wait for a few minutes and observe what happens to the substances added to the’ water.
Note down your observations in the following table.
































0 Comments